The failure rate for medical device startups can be as high as 90%. The question is, why do so many fall short? Is it due to funding challenges, the complexity of the technology, over-commitment coupled with under-delivery, a lack of understanding of the competitive landscape, or perhaps a shortage of engineering experience and manufacturing capabilities?
Embarking on a journey from concept to market-ready product involves navigating a complex landscape of challenges and opportunities. In this three-part series, we’ll explore the intricacies of product development through commercialization. We begin by delving into the proven Optikos product development process, known for its effectiveness in guiding products from ideation to realization. In Part 1, we will focus on Pre-Development, the first stage of the Optikos Medical Device product development process as shown in Figure 1.
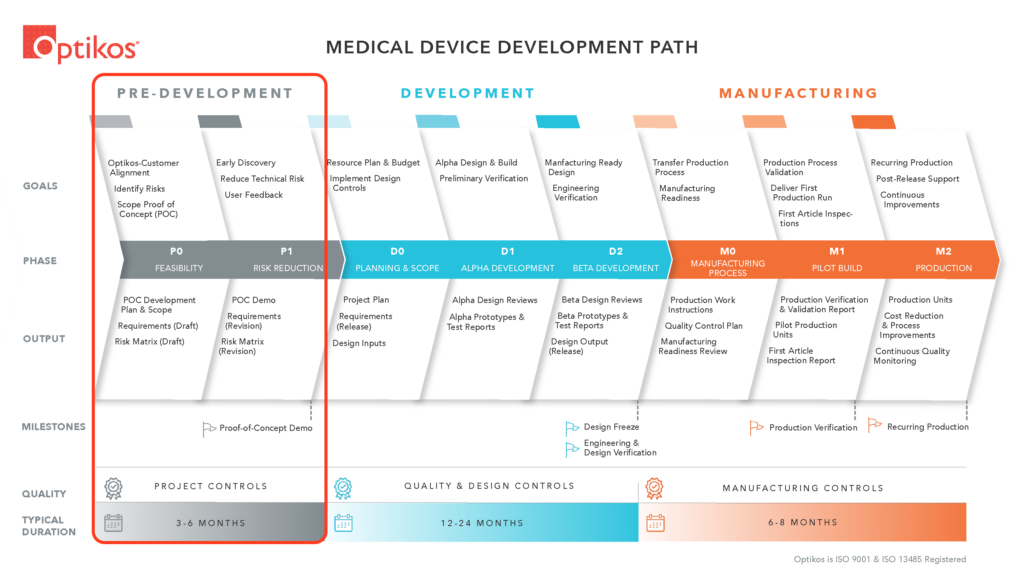
Transitioning an idea from a sketch on a napkin to a fully developed, commercialized product is fraught with challenges. Among these challenges, several key areas often stand out:
- – Regulatory Hurdles
- – Funding Issues
- – Team Expertise
- – Market Fit and Value Proposition
- – Technological Challenges
- – Quality Control
One of the most significant issues is the lack of a clear, detailed pathway from concept to completion.
In addition, each company is unique, bringing its own set of challenges. However, a common thread among successful companies is the use of an effective and proven product development process. This process should provide more than just a framework for a new product; it must guide the management of budget, timeline, resources, and milestones while ensuring compliance with the regulatory framework.
It’s also crucial to highlight the importance of assembling a team of industry professionals. Building a team with the right skill sets and engineering disciplines is essential for successful product commercialization. The development process is often lengthy, taking months or even years, depending on funding and timelines. Engaging with industry experts, such as Optikos, can help streamline the development timeline, potentially accelerating the time to market. For engineering directors and project managers, recognizing and addressing these challenges early in the process is key to navigating the complex landscape of medical device development.

Within the Pre-Development stage of the Optikos development process (Figure 2) there are two key phases: Phase 0 (PO): Feasibility and Phase 1 (P1): Risk Reduction.
Phase 0 (P0): Feasibility focuses on determining the technical and commercial viability of the project. This phase assumes that fundamental research is complete and that the customer’s objectives are clearly defined. It sets the foundation for the development cycle by establishing clear program expectations and identifying potential risk, whether technical, budgetary, or timeline-related
Phase 1 (P1): Risk Reduction aims to address and mitigate the identified risks. By resolving technical issues early on, this phase helps to inform and refine product specifications, ensuring that the development process is aligned with both technical requirements and business goals.
Both phases are crucial for setting the direction of the entire development process, providing a structured approach to managing risk, and ensuring that the project is positioned for success from the outset.
As mentioned, Phase 0 involves assessing the viability of a project by setting clear inputs, goals, and outputs— a process that will be consistent across all subsequent phases. Companies may enter this phase from different starting points:
- – Company A might focus on patent research, generating concepts based on findings, and conducting preliminary risk assessments.
- – Company B could be developing initial optical or mechanical designs, utilizing simulations to gauge feasibility and mitigating risk early on.
This phase is critical for aligning on project scope and determining the potential for success before significant resources are committed.
In Phase 0, Optikos typically completes the project kickoff and initial work within four to six weeks, involving one to two full-time engineers (FTEs). Meeting deadlines is crucial in this phase, and selecting the right mix of resources is key to maintaining efficiency. The correct blend of engineering expertise helps minimize lead times, ensuring the project stays on track and within budget. Conversely, an inappropriate mix of experience can extend timelines and increase costs, leading to higher overall investment.
Phase 0 Example – 3D Stereo Imaging Feasibility
In this example, our team completed Phase 0 for a customer developing a new 3D stereo-imaging system for surgical use. This system, designed to allow “heads-up” surgeries, required a thorough assessment to ensure it could meet market and product requirements. The entire process took 10 weeks.
Challenges Identified:
The company had already spent about 18 months developing optical and opto-mechanical models. However, several critical design issues were identified:
- Imaging Performance: The system wasn’t meeting the required imaging specifications.
- Working Distance: The distance between the patient and the digital microscope was off.
- Size and Weight Constraints: The system was too large and heavy.
The Optikos Approach:
Given the urgency to move into prototyping, Optikos collaborated with the customer using our proven phased process. Together, we developed a statement of work to address these issues, ensuring the trade space designs met all necessary specifications and could advance to the next development phase.
This Phase 0 work set the foundation for a smooth transition into Phase 1, where the design would be further refined and prepared for prototyping.
Phase 0 Tasks:
- Measure the Optical Performance of Off-the-Shelf Stereo Microscopes. Lens and System MTF measurements will be collected from the microscopes for multiple magnifications and field points using Optikos’s state-of-the-art OpTest® metrology system. There is an expectation that continued measurements of the proof-of-concepts (POCs) will be required. A test report will detail the results and comparisons of the microscopes.
- Evaluate Optical Trade Space. Multiple optical designs will be created and evaluated to assess the impact on variations in image quality, disparity, working distance, magnification/FOV ranges, size, and other performance metrics.
- Analyze the Trade Space of Opto-Mechanical Design. An evaluation of the proof-of-concept design will be performed by looking at current proof-of-concept (POC) performance, expected needs for new designs, and the size, weight, cost, and performance trade space.
- Measure Proof-of-Concept (POC) Optical Thermal Performance. Stereo Objective (STO) optical performance will be evaluated across a temperature range (20°C to 60°C) on an Optikos OpTest® bench by our IQ Lab™ Services engineers. The performance of a completed POC module will also be evaluated at an elevated temperature.
- Measurements and Analyses will be used to provide specification suggestions to the customer for the Prototype Design Phase (Phase 1 and beyond).
- Investigate Illumination Layouts. Evaluate angle sensitivities and associated requirements using simulations in Zemax.
- Generate Program Risk Table, refer to Figure 3.
| Risk | Profitability | Mitigation |
| Timeline / Schedule | High | Before this phase, a trade space analysis is conducted to help customers quickly finalize and freeze the optical specifications. The project will run optical and mechanical development projects concurrently ending this Phase with a Preliminary Design Review. To assist with the overall project timeline and phases beyond the scope of efforts, Optikos will identify and purchase long lead items as soon as reasonably possible to minimize schedule slip in subsequent phases of the project. Time is budgeted for frequent communications with the customer team with expectations for multiple weekly interactions to allow for fast decisions and minimize the chance of surprises. |
| Technical Performance | Medium | Optikos has structured the proposed development project to include multiple specifications and design freezes. Phase 0 and the proof of concept development have helped to reduce technical risk in the project. Also while Optikos will assign a core engineering team to the project, Optikos always leverages the collective knowledge and experience of the full engineering and technical staff at Optikos for all projects. |
| Cost | Medium | Optikos has developed a detailed project plan to estimate the expected level of effort to reach optical and mechanical design freezes. Constant communication with the customer and periodic programmatic updates will ensure that potential cost overruns or scope increases are handled in a timely fashion. |
During the 10-week Phase 0 Optikos effectively executed all critical tasks, enabling the customer to successfully pass their internal phase gate. This collaborative effort between Optikos and the customer paved the way for a smooth transition into Phase 1. By entering this phase, the team ensured that the project’s trade space would continue to yield solutions that are viable in the market.
Phase 1 – 3D Stereo Imaging Risk Reduction
Phase 1: The Risk Reduction phase, a key stage in the Pre-Development process, is critical for advancing a commercial product. This phase focuses on risk mitigation, refining the product architecture, and building prototypes to validate the technology’s market viability.
Upon completion of Phase 0 of the customer program, the Optikos team identified high-risk areas within the design. These risks were documented and shared with Optikos’s customer in a comprehensive risk table (see Figure 2).
Now, in Phase 1, the focus shifts to addressing and reducing the risk identified in Phase 0. This involves exploring and evaluating the performance of the selected design architectures. Throughout this phase, Optikos adheres strictly to its Quality Management System, ensuring compliance with ISO 13485 and 21 CFR 820 (FDA) regulations, particularly as they pertain to the design and development of medical devices.
To ensure the success of Phase 1, it’s essential to establish a formal specification document that both parties agree upon. This document serves as the cornerstone for all design decisions, but its importance extends beyond that. It plays a crucial role in managing scope creep, timelines, and budgets, and maintaining strong relationships with all stakeholders. By locking down this specification early, Optikos sets clear expectations and reduces risks, helping to keep the project on track and aligned with the project goals.
The Phase 1 effort will include a design review of the deliverables. In this example, the Optikos team provided a detailed list of outputs that would be reviewed upon completion of work:
- – Optical Design Package
- – Mechanical Design Package
- – Preliminary Design Review
- – Periodic Review Meetings
The Optikos team partnered with the customer to complete Phase 1 of the project, achieving significant milestones within a compressed timeline. Over 14 weeks, the collaborative effort, involving four full-time engineers, accumulated approximately 1,000 hours of focused work.
Key Achievements:
Risk Mitigation and Technology Selection: The Optikos team played a crucial role in reducing project risk by carefully down-selecting a market-viable 3D imaging technology. This strategic decision was pivotal in guiding the project toward a successful outcome.
Rapid Prototyping: Within 14 weeks, the team successfully built 10 proof-of-concept (POC) prototypes. These prototypes were essential in validating the technology’s market viability.
This rapid and efficient execution allowed the customer to pass their internal phase gate, providing senior leadership with the confidence to proceed, backed by tangible proof of the technology’s potential. The collaboration not only accelerated the development process but also ensured that critical project milestones were met, demonstrating the value of leveraging engineering consultants in high-stakes projects.
In conclusion, the Pre-Development stage, encompassing Phases 0 and 1, is crucial for any organization aiming for successful commercialization. These phases involve setting clear goals, defining tasks, identifying risk, and establishing deliverables—all aligned with a customer’s project timeline and funding. Proper management of these elements will significantly influence the success of a customer’s project and pave the way for future achievements.
This efficient process validated the technology’s market viability. Partnering with a valued consultant like Optikos enhances a project’s chance of success by leveraging Optikos’s proven methodologies and technical skills.

